Research
Our research uses mimicry-associated butterfly wing color patterns as a model for adaptation to address three interrelated questions: A) What are the developmental and genetic mechanisms of adaptation? B) How do these adaptive mechanisms evolve? And, C) How are adaptive phenotypes maintained despite maladaptive gene flow?
Some of the ways our research addresses these questions are highlighted below.
Evolution of wing pattern mimicry in Heliconius butterflies
Adaptive warning coloration in Heliconius butterflies is a well-known case study for the process of adaptive phenotypic divergence, where individual species have co-diversified into dozens of color pattern morphs to maintain regional mimicry color patterns that deter predation. Previous studies of red wing color pattern variation in Heliconius indicated that a single Mendelian locus around the transcription factor optix controls all of the red color pattern divergence in Heliconius.
My work on the evolution of Heliconius wing pattern mimicry has aimed to identify the genetic mechanisms that underlie wing phenotype evolution. We found that derived red wing pattern phenotypes in the Amazon (Radiate morph, above) evolved via evolution of at least five cis-regulatory loci that appear to contain both enhancer (gene activator) and silencer (gene repressor) activity (PNAS 2019). In a subsequent study, we also identified dozens of loci directly regulated by Optix that associate with red wing pattern adaptation and maintain wing pattern morphs along hybrid zones (Science Advances 2020). These results highlight the importance of epistasis in diversification and suggests many “Mendelian” traits may, in fact, have evolved through a multigenic process.
My ongoing work in this area includes several collaborative projects mapping the cis-regulatory architecture of wing pattern phenotypes (e.g. melanic wing patterns) and functional tests of downstream genetic variants associated with mimicry pattern evolution.
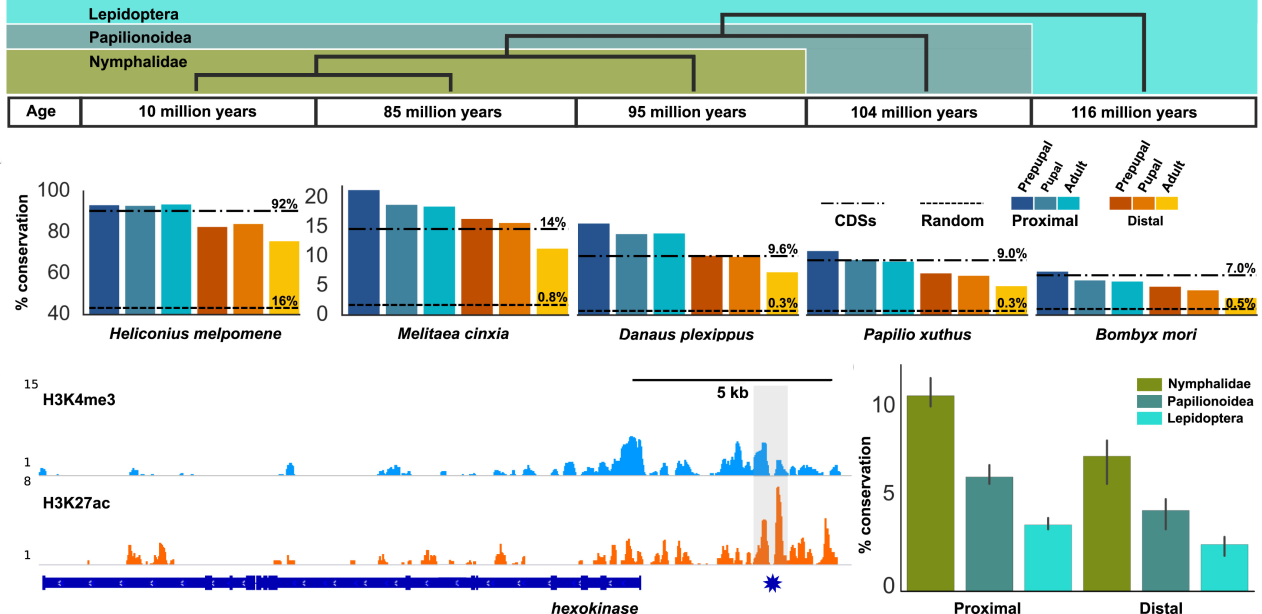
Mechanisms of functional genomic divergence in Lepidoptera
Determining the mechanisms and evolutionary processes that govern divergence is critical to generating and testing models of genome evolution. My work in this area has focused on how cis-regulatory evolution drives genome divergence.
I studied CRE evolution in three populations of H. erato butterflies to test whether many loci differentiate stable populations outside of hybrid zones (MBE 2018). I found that CRE variation between H. erato populations is ubiquitous throughout the genome and often under strong selection. While specific loci may separate morphs at population boundaries, this result indicates that the ability for gene flow to penetrate population centers is limited and many loci contribute to overall population divergence. To study how mechanisms of gene regulation influence species divergence, I studied gene regulatory evolution across 115 million years of butterfly evolution (Cell Reports 2016). We found that enhancers evolve much faster than promoter sequences. Interestingly, however, promoter and enhancer loci were shown to evolve at similar rates when controlling for pleiotropy, providing a developmental basis for observations of rapid enhancer evolution.
I recent investigated whether functional DNA elements such as CREs and exons are resistant to strong neutral processes like changes in recombination rate (MBE 2021). Leveraging 10 chromosome fusion events in Heliconius that drove a decrease in recombination rate among fused chromosomes, we indeed found that functional DNA elements were less impacted by changes in recombination rate when compared with non-functional DNA. This result is consistent with my previous observation that adaptive cis-regulatory divergence is pervasive and suggests that primarily non-neutral processes govern functional genome evolution.
My current work on the mechanisms of genomic divergence include several collaborations to study the role of cis-regulatory divergence in tissue-specific evolution in Heliconius butterflies.
Genetic architecture of sex-biased gene expression and sexual dimorphism
Adaptive evolution of sex-linked traits poses a series of unique problems for understanding diversification not seen in evolutionary divergence between populations: Given a mostly shared genome, what is the genetic architecture of sex-linked traits? What processes drive selective separation of sexes? How does a species resolve sexually antagonistic selection?
My future research aims to integrate computational analysis with comparative population and functional genomic datasets to investigate: A) the genetic architecture of sex-biased gene expression in sexually dimorphic butterflies, B) the evolution of sexually dimorphic phenotypes, and C) sex-linked traits as mechanisms of population divergence and speciation.
A chromosome-level genome assembly for Dryas iulia was just released (GBE 2021). I am now investigating whether mechanisms of sex-biased gene expression evolve via local, independent selection at many loci or through trans-regulatory cascades, where changes in upstream regulatory network nodes can produce large-scale changes in gene expression in downstream network components.